Eyenovia is the Optejet® company
NASDAQ: EYEN
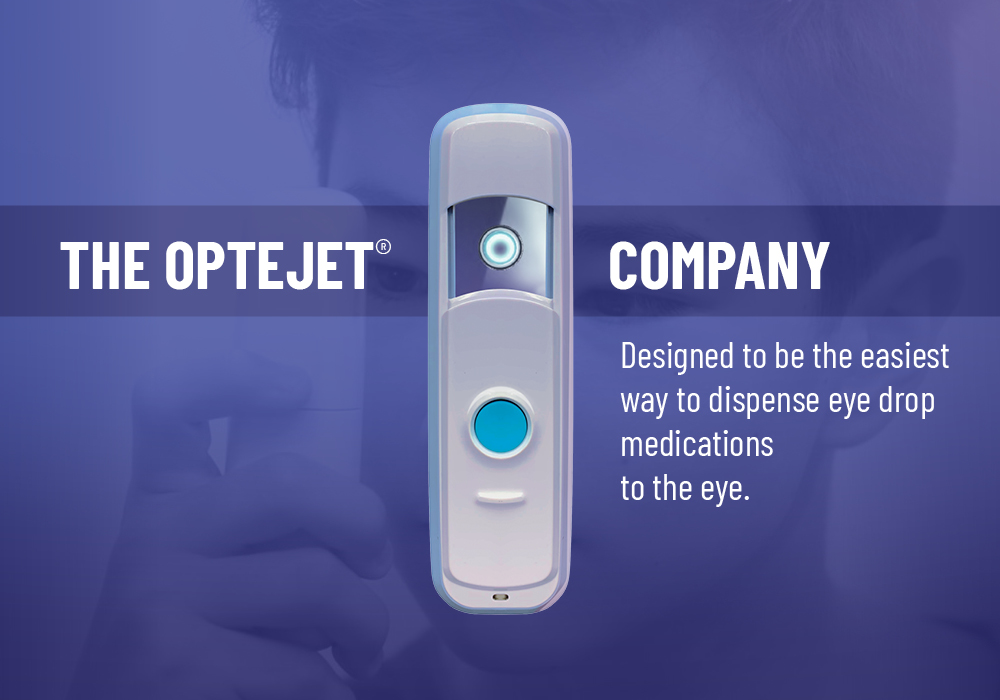
A public eye care company focused on improving the usability and administration of eye medications with our Optejet® device for use by our patients and their caregivers.
The Optejet® is the only FDA-approved ophthalmic spray alternative to eye drops.
Pipeline
Our pipeline candidates are designed to address the issues of conventional eye drop bottles delivering the appropriate dose and a better patient experience. More
MicroPine
Eyenovia’s lead pipeline candidate is MicroPine for the prevention of myopia progression in children. If approved, MicroPine will be the first drug-device combination product for this condition. The Optejet could enable more children to self-administer atropine. More